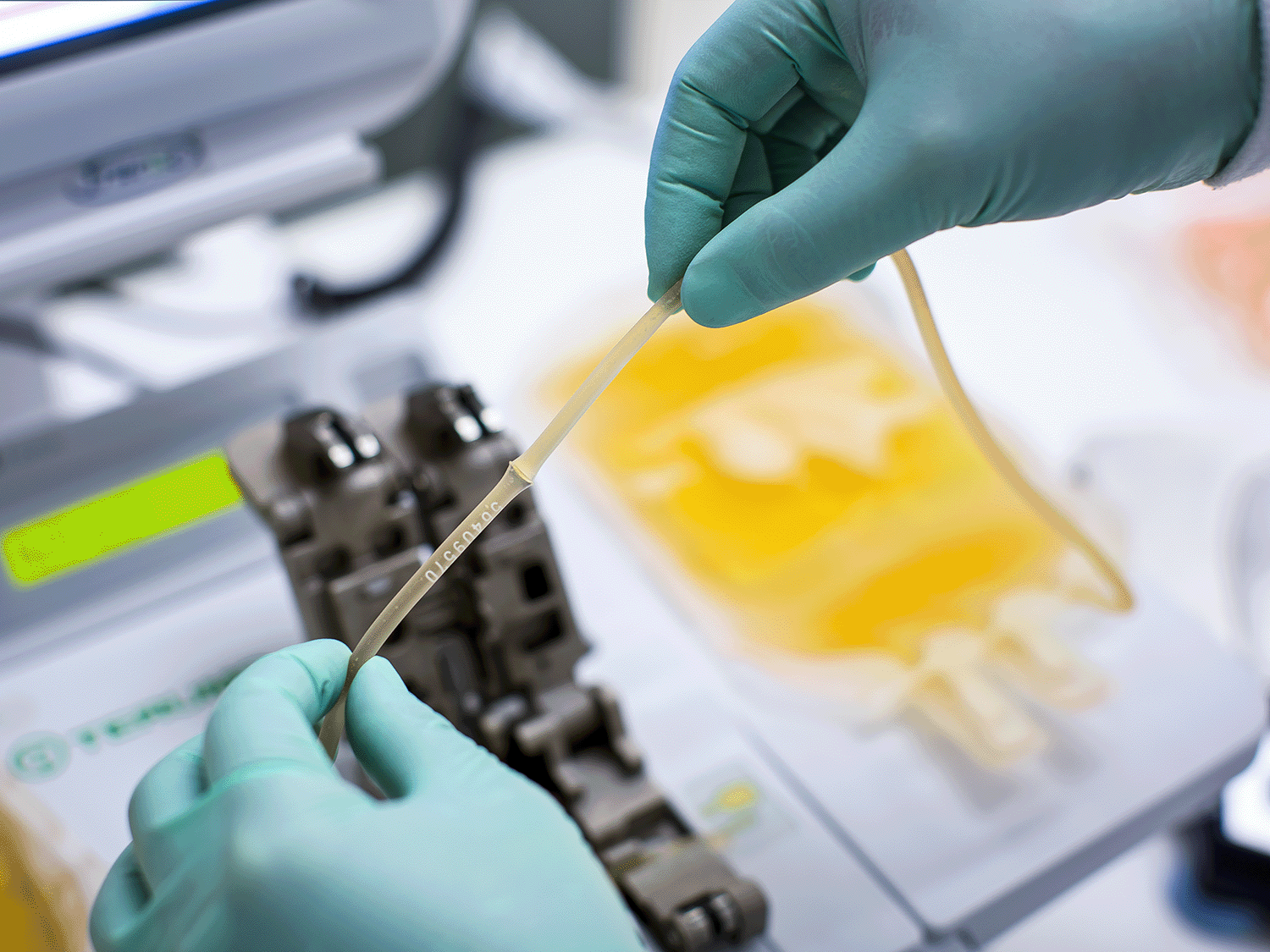
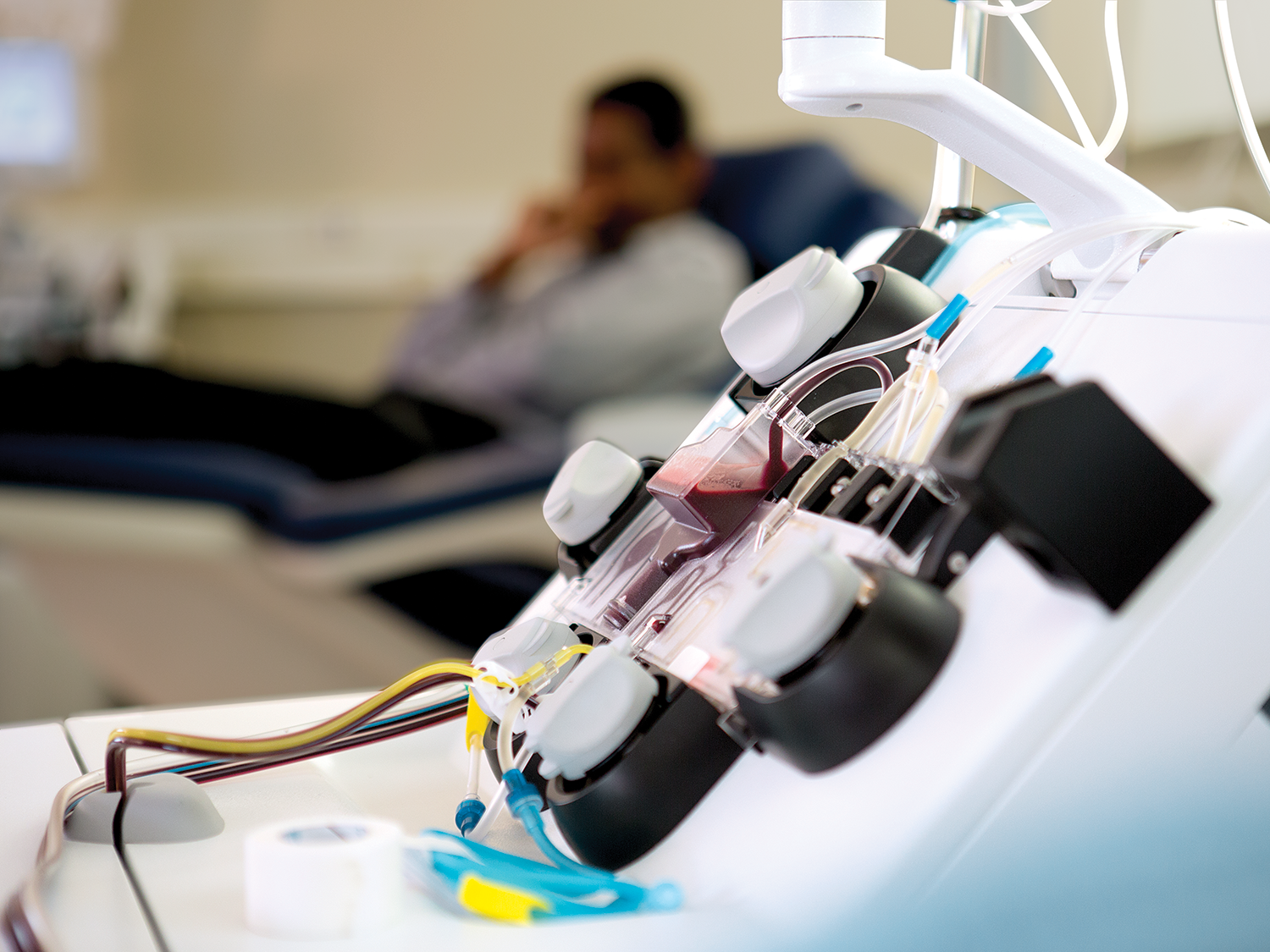
强大、可靠而拥有巨大的潜力
Mirasol PRT 系统是在人类临床试验中显示可减少输血传播性感染 (TTI) 疾病的一种 PRT 系统,可使疟疾 TTI 降低 87%。1
为更好的血液去做正确的平衡
我们相信,在保证病人需要的血液成分供应的同时,更好的血液安全能够提供效用与易用之间的正确平衡。Mirasol PRT 系统是一个简单的系统,其能够提供更好的病人关怀,减少了与救命的血液制品相关的风险。
Mirasol PRT 系统
您比任何人都清楚 - 这是充满挑战的时代。Mirasol PRT 系统使您可以通过优化血液制品成本、效用和安全性之间的平衡,从而满足您的业务需求。2,3
值得信赖的伙伴
Mirasol PRT 系统系血液成分技术领域全球综合方案提供商 Terumo BCT 出品。
工作原理
提高血液安全性的正确途径:安全、简便、有效
Mirasol PRT 系统使用核黄素(维生素 B2;一种无毒性无致突变的化合物)结合特定的紫外 (UV) 光谱照射来灭活所采集血液制品中可能存在的病毒、细菌、寄生虫和白细胞。
Mirasol PRT 系统由三个主要部分组成:
- 一次性使用套件 - 包括一个照射/储存袋和无菌核黄素溶液
- Mirasol 照射器 - 为 Mirasol 处理提供紫外光照射及震荡
- Mirasol 管理软件 - 集成和管理数据报告及存储
在处理过程中,将血液制品与核黄素溶液混合,并置于照射器中,使之暴露于紫外光下。无需去除核黄素或其光产物;照射后,经处理的制品即可进行输注或放置入库。
流程
血小板、血浆和新鲜全血都是三个简单步骤
使用Mirasol处理的血小板的临床经验
病原体减少评估和预测分析评分(PREPAReS)临床试验是迄今为止最全面的临床试验,证明了Mirasol处理的血小板不劣于传统处理。此外,泰尔茂血液与细胞技术公司举办了题为“使用Mirasol处理的血小板的临床经验”的网络研讨会,由输血医学和血液安全研究和实践的国际领导者Jeffrey McCullough博士主讲。
资料
如需操作手册和使用说明,请访问我们的 eIFU 网站:Terumo.eifu.com。更多信息请访问 Help.TerumoBCT.com。
了解 Mirasol 如何提升您血液中心的血液安全性。
免责声明和说明
该产品在中国暂未上市
Mirasol PRT 系统符合医疗器械指令。
Mirasol PRT 系统尚未获批在美国销售,仅在选定市场有售。
Mirasol PRT 系统已获得血小板、血浆和全血应用的 CE 标志。
- Allain JP, et al. Blood. 2015;126(23):770.
- Custer B, et al. Transfusion. 2010;50(11):2461-2473.
- Agapova M, et al. Transfus Med Hemother. 2015;42(3):158-165.
- Mundt JM, et al. Photochem Photobiol. 2014;90(5):957-964.
- Reddy HL, et al. Transfus Med Rev. 2008;22(2):133-153.
- Goodrich RP, et al. Chapter 5: The Antiviral and Antibacterial Properties of Riboflavin and Light: Applications to Blood Safety and Transfusion Medicine. In: Flavins: Photochemistry and Photobiology. Royal Society of Chemistry; 2006.
- Jimenez-Marco T. The benefits of working with Mirasol PRT. Presented at: 4th Mirasol International Users Meeting; Estoril, Portugal; 2014.
- Bautista AM. Logistics and economical aspects of Mirasol implementation. Presented at: 5th Mirasol International Users Meeting; Palma de Mallorca, Spain; 2015.
- Jimenez-Marco T, et al. Transfus Med Hemother. 2014;41(3):172-175.
- Marschner S, Goodrich R. Transfus Med Hemother. 2011;38(1):8-18.
- Keil SD, et al. Transfusion. 2015;55(7):1736-1744.
- Keil SD, et al. J Vis Exp. 2015;102:52820.
- Yañez Izquierdo M, et al. Transfusion. 2009;49(suppl 3):84A.
- Yañez M, et al. Vox Sang. 2011;101(suppl 1):148.
- Coene GC, et al. Vox Sang. 2011;101(suppl 1):188-189.
- Mirasol Clinical Evaluation Study Group and RP Goodrich. Transfusion. 2010;50(11):2362-2375.
- Trakhtman P, et al. Transfusion. 2016;56(suppl. 1):S24-S28.
- Kaplan A, et al. Transfus Apher Sci. 2015;54(2):248-252.
- Fast LD, et al. Transfusion. 2011;51(7):1397-1404.
- Fast LD, et al. Transfusion. 2013;53(2):373-381.
- Goodrich RP, et al. Transfusion. 2009;49:1205-1216.